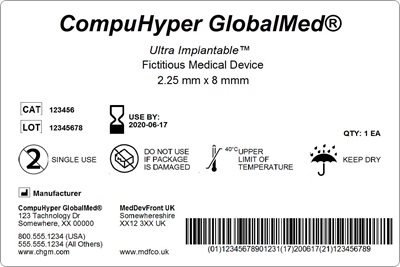
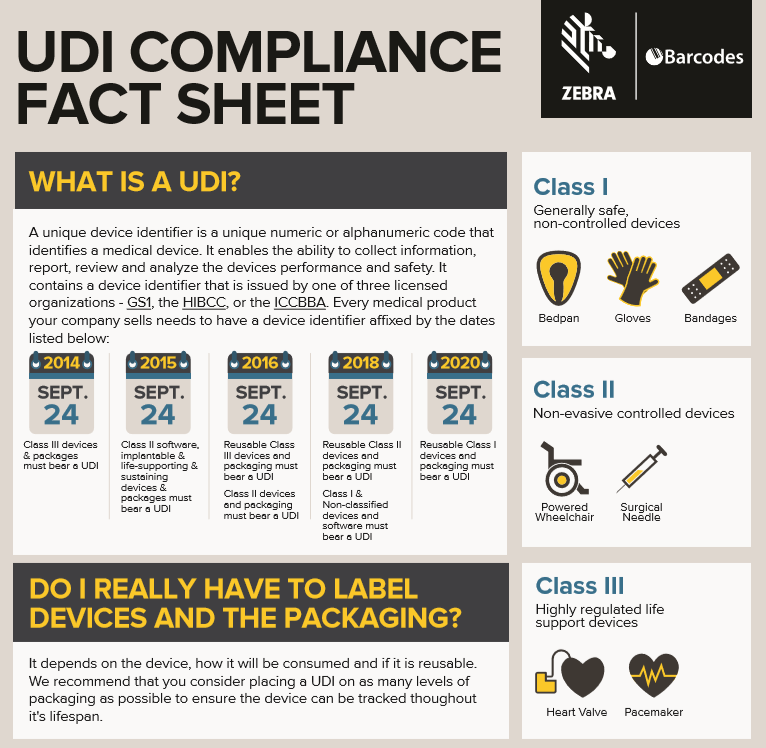
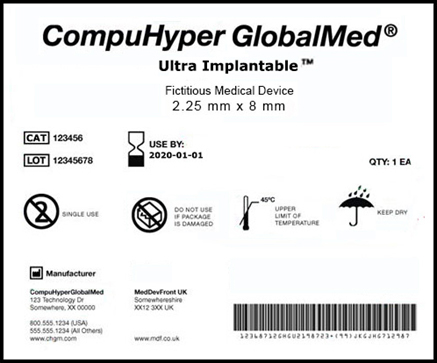
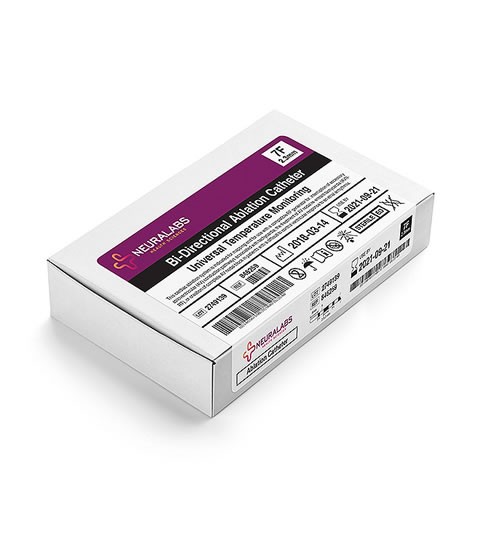
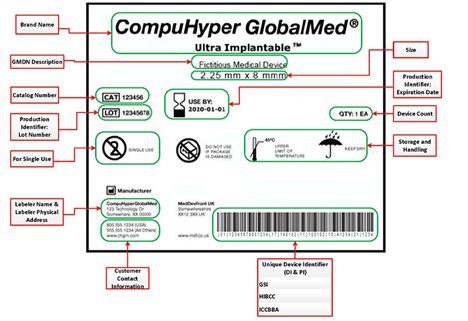
Towards a Logic-Based Extension of a Relational Software Tool for Coherent Technical Documentation of Medical Devices - In Compliance Magazine

Connected systems in label management: How integrated software saves time, money and lives - Medical Device Network
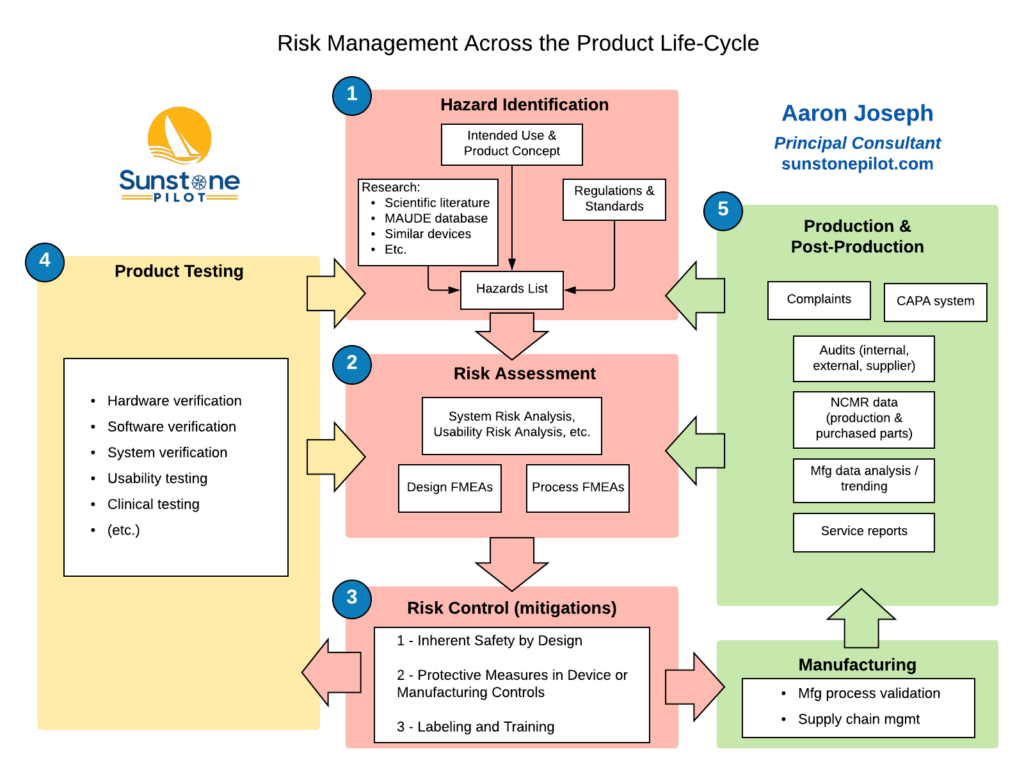
![QMS for Software as a Medical Device [SaMD] Lessons Learned from a Quality Perspective - PDF Free Download QMS for Software as a Medical Device [SaMD] Lessons Learned from a Quality Perspective - PDF Free Download](https://docplayer.net/docs-images/42/17134037/images/page_2.jpg)
QMS for Software as a Medical Device [SaMD] Lessons Learned from a Quality Perspective - PDF Free Download